
![]()


Half of the yearly administered 16 billions injections are considered unsafe, particularly on the practices in the developing countries (WHO Injection Safety, Quality of Immunization Services, August 28, 1998). The habit to reuse the injection devices for multiple injection administrations have caused paramount cross infection risk to the society for infectious diseases such as Hepatitis B or C or HIV. As a result, WHO has mandated all injection devices for immunization programs worldwide must use Auto Disable Syringes to be bundled with Safety Disposal Boxes.
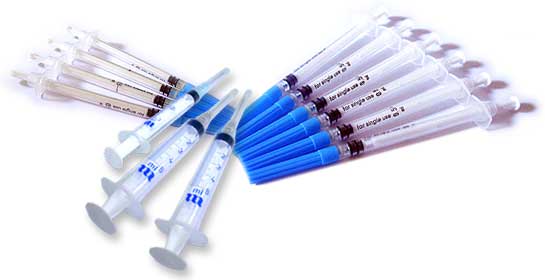
Medibest is the appointed Sole Agent and acts as Strategic Partner for Abu Dhabi Medical Devices & Co. (Medeco), a joint venture company between ADNIP Group and Pharmaplan International GmbH, Germany , for its Far-East marketing and distribution activities. Apart from manufacturing high quality Conventional Hypodermic Syringes with different type of sizes and IV-Catheters, Medeco manufactures a WHO approved and recommended Medeco® Inject AD, 0.5ml, Auto Disable Syringe for Immunization, which is manufactured within the guideline of WHO Standard E8/DS.1 (October 1999 Edition), ISO 13485, ISO 7886-3, ISO 9001:2000 and CE Certification. It has been WHO approved and included in the WHO Product Information Sheets (PIS) with WHO PIS Code E8/22 and in WHO Product Quality and Safety (PQS) with WHO PQS Code E8/04. These syringes are manufactured in modern manufacturing facility, which is certified with the WHO GMP Certification.
![]()

Reuse Prevention Syringe (RUP) is basically a syringe intended to avoid re-use as well as disable syringes (ADS).Medeco reuse prevention syringe inject 2/3 ml and 5 ml RUP are indicated for use in immunization programs such as Medeco inject 0,5ml ADS AD and AD Medeco inject 0,5ml (BCG) ADS, also in use in the injectable contraception or any other curative medicine.